Certification
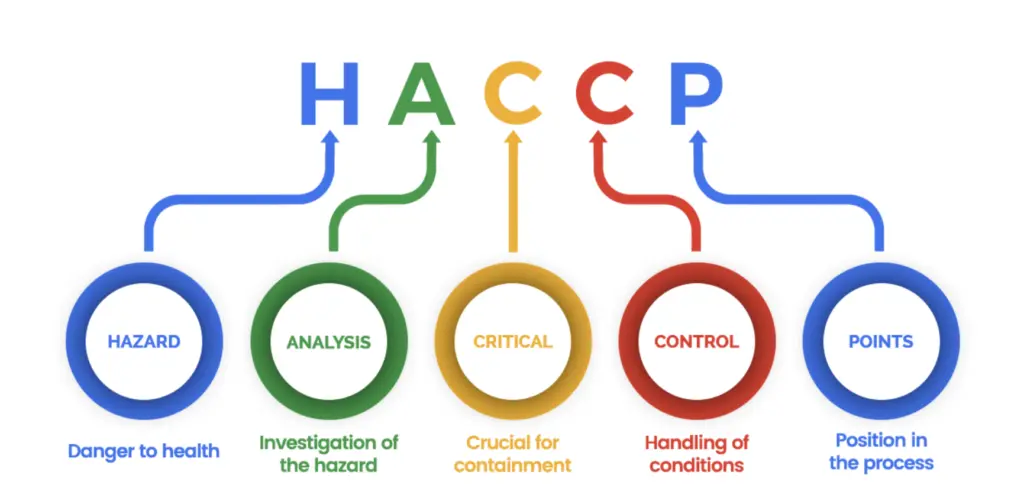
Medela Pharma operates under a robust framework of international certifications, reflecting its unwavering commitment to quality, safety, and regulatory compliance. The company is authorized to release pharmaceutical products under Swissmedic certification, ensuring adherence to stringent Swiss and international health authority standards. For its extensive work in the field of food and dietary supplements, Medela Pharma maintains HACCP (Hazard Analysis and Critical Control Points) certification, which guarantees that all processes meet globally recognized food safety principles.
Further reinforcing its dedication to quality in the nutraceutical space, Medela Pharma is GMP certified under the NSF/ANSI 455-2 standard, specifically for dietary supplements, ensuring consistent product quality and manufacturing practices. The company also complies with TGA (Therapeutic Goods Administration) health and safety regulations, allowing it to supply products to the Australian market with confidence. Additionally, Medela Pharma is FDA registered, assuring that its facilities and processes meet regulatory requirements for safety and efficacy. These certifications collectively position Medela Pharma as a trusted, globally compliant partner in pharmaceutical and supplement manufacturing.
Partner with Medela Pharma to Accelerate Your Path from Discovery to Commercialization.
Let’s work together to deliver innovative, high-quality solutions across the pharmaceutical lifecycle